How Can a Redox Reaction Be Used as a Source of Electrical Energy
Chemical reactions that can generate electricity are called REDOX reactions. REDOX reactions occur in the batteries you usually buy to ability your electronic devices. REDOX reactions consist of 2 types of reactions. One is called reduction, while the other is called oxidation. When yous combine the Ruby-red part of reduction to the Ox part of oxidation, y'all volition get the acronym REDOX, which means reduction and oxidation reactions. Why do these reactions generate electricity? If you consider electricity simply to be the menstruation of electrons, so you lot can say that in REDOX reactions some chemicals are naturally inclined to lose electrons while others are naturally inclined to accept electrons. As a result, when these chemicals are kept in separate containers and connected by a wire, electrons usually catamenia through the wire from one chemical to the other, producing electricity every bit a outcome.
What is reduction in redox reactions?
When an atom accepts an electron, chemists normally say that the atom has undergone reduction. Another mode of maxim the same thing is that the atom has been reduced. Therefore, REDUCTION is defined equally the gain of electrons.
What is oxidation in redox reactions?
When an atom loses an electron, chemists usually say that the cantlet has undergone oxidation. Some other manner of saying the aforementioned matter is that the atom has been oxidized. Therefore, OXIDATION is divers as the loss of electrons.
Every bit you can tell, in redox reactions, reduction cannot occur without oxidation and oxidation cannot occur without reduction. Thus, the two reactions must occur together to generate electricity. For this reason, REDOX reactions are sometimes chosen electron transfer reactions.
An example of a redox reaction includes:
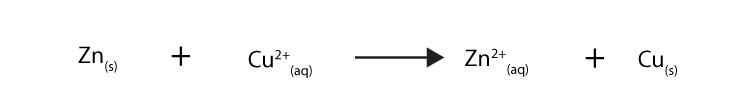
Now, from the equation higher up, how can yous tell that electrons are being transferred as reactants plow into products? Since the chemical equation includes the charges of the chemicals, you tin tell by tracking how the charges of each chemical are changing on the reactants and products side of the chemical equation.
For instance, since the accuse of Zn goes from neutral to +2, it follows that Zn must have lost ii electrons. And since the charge of Cu goes from +2 to neutral (0), it follows that Cu must have gained 2 electrons. Therefore, you lot tin say that Zn has been oxidized to Zn2+ by losing 2 electrons, while Cutwo+has been reduced to Cu by gaining two electrons.
Now, imagine that you have been given the post-obit balanced chemical equation:
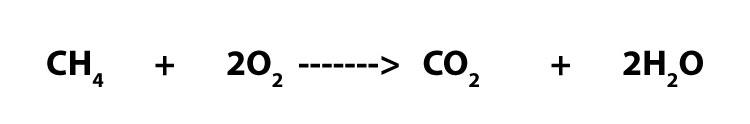
How do you tell that electron transfer is occurring? Equally you lot can see, unlike the previous one, the chemicals in this equation are all neutral molecules. This ways that it will be difficult to tell without you doing a little bit of electron bookkeeping. To larn more nigh this electron accounting, click here.
Source: https://masterconceptsinchemistry.com/index.php/2019/01/27/what-types-of-chemical-reactions-generate-electricity/
แสดงความคิดเห็น for "How Can a Redox Reaction Be Used as a Source of Electrical Energy"